infectious Agent: yellow fever virus is a single-stranded RNA virus.
Yellow fever virus(CDC).
mode of Transmission: vector-borne transmission by mosquito(Aedes or Haemagogus spp.)
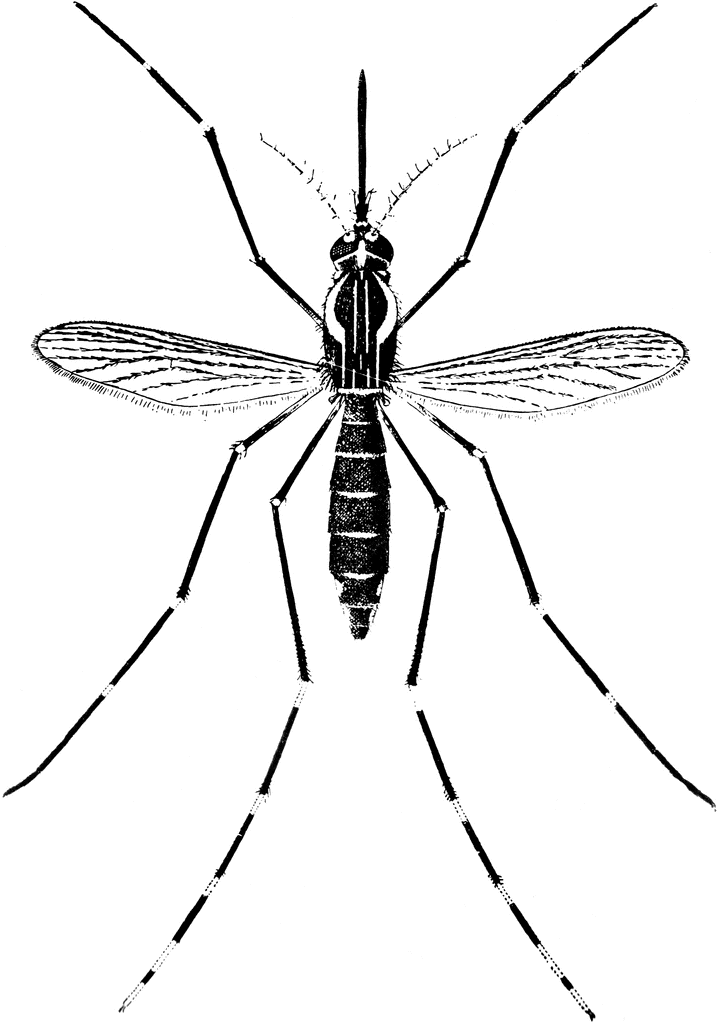
Adults of the yellow fever mosquito Aedes aegypti, a typical member of the subfamily Culicinae. The male on the left, females on the right. Note the bushy antennae and longer palps in the male.
Reservoirs :nonhuman and human primates.
transmission cycles:
1. sylvatic(jungle)(human primates-mosquito-human)
2. intermediate(savannah) (monkeys-humans.human-human via tree hole-breeding Aedes supp.)
3. urban(human-human via urban mosquitoes Ae.aegyti)

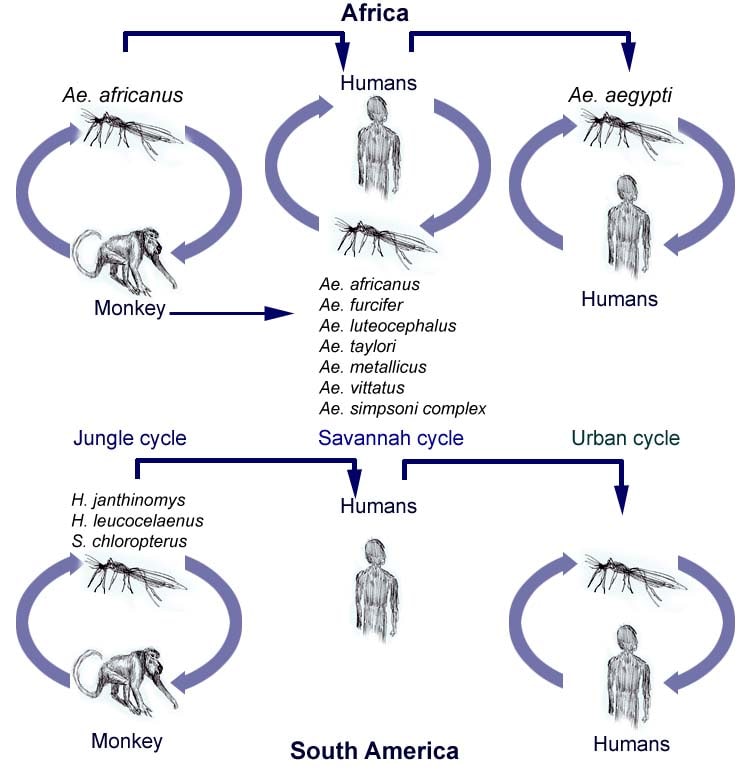 Transmission cycles of yellow fever in Africa and South America. Adapted from Annu Rev Entomol. 2007. 52:209-29.
Transmission cycles of yellow fever in Africa and South America. Adapted from Annu Rev Entomol. 2007. 52:209-29.
Human with YFV experience the highest levels of viremia and bloodborne transmission can also occur.
Occurence
Sub-Saharan Africa and Tropical South America

Africa: natural immunity accumulates with age. infants and children are at great risk for disease.(savannah)
South America: young men are exposed to mosquito vectors through their work in forested or transitional areas.(jungle)
urban yellow occurs periodically in Africa and sporadically in the Americas.
Risk for Travelers
factors:
1. immunization status;
2. location of travel;
3. season;
4. duration of exposure;
5. occupational and recreational activities while traveling;
6. local rate of virus transmission at the time of travel
7. "epidemiologic silence" attention
Africa
seasonal,with an elevated risk during the end of the rainy season and the beginning of the dry season(usually Jul.-Oct.)
South America
rainy season(Jan.-May,with a peak incidence in Feb. and Mar.)
Clinical presentation
incubation period is typically 3-6 days.
initial illness presents: nonspecific influenza-like syndrome with sudden onset of fever, chills, headache, backache, myalgias, prostration, nausea, and vomiting.
progress: toxic form of the disease like jaudice, hemorrhagic symptoms, and shock and MOF.
overall case fatality ratio is 20%-50%.

Diagnosis
1)clinical features,
2)places and dates of travel
3)activities.
4)lab diagnosis: virus-specific IgM and IgG
early in the illness, rna can be detected in serum samples by NAAT but it is usually undetectable when more over symptoms are recognized.
Treatment
No specific treatment,
just a symptomatic treatment like rest, fluid, use of analgesics and antipyretics. avoid aspirin or other nonsteriodal anti-inflammatory drugs which may increase the risk of bleeding.
Preventive measures for travelers
personal protection measures
NO DRUGS for preventing infection are available.
The best way is to avoid mosquito bites.
1. use repellent
1) DEET(N,N-diethyl-meta-toluamide).
Products containing DEET are intended to be applied to the skin. Studies indicate that repellents containing DEET at concentrations >20% provide the longest periods of protection against mosquito bites but even low (5-10%) concentrations protect for 1-3 hours.

2) Picaridin (chemical name, 2-(2-hydroxyethyl)-1-piperidinecarboxylic acid 1-methylpropyl ester)
It is a colorless, nearly odorless liquid active ingredient that is recommended by the Centers for Disease Control and Prevention (CDC) as an alternative to DEET. Picaridin is available in Cutter™ and OFF™ products.
Lab and field studies of products containing picaridin (10-20%) indicate good protection with this compound (Barnard and Xue 2004, Barnard et al. 2002; Naucke et al. 2007; a summary of 14 trials can be found in Frances 2007). Products with lower concentrations (7.5%) are also on the market, and these may need field testing.

3) based on IR3535
IR3535 is recommended by the Centers for Disease Control and Prevention (CDC) as an alternative to DEET. IR3535 is a synthetic insect repellent structurally similar to a natural amino acid, beta-alanine and is classified as a biopesticide by the EPA. This compound has been used as a mosquito repellent in Europe and Asia for 10-20 years (Marchio 1996) and was approved by the U.S. EPA in 1999. IR3535 is currently available in the Avon Skin-so-soft line of mosquito repellents (see below).
Pucetti (2007) summarizes the results of 13 laboratory and field trials. In two recent field studies, an IR3535 containing repellent provided good protection (Naucke et al. 2007, Thavara et al. 2001). A lab study using Avon Expedition insect repellent, 20% IR3535, resulted in protection similar to 20% DEET (Cilek et al. 2004). However, in other laboratory trials, protection was short-lived, ranging between 10 and 60 minutes (Fradin and Day 2002). This study used a product containing a concentration of 7.5% IR3535 (Avon Skin so soft Bug guard plus 7.5%). Similarly Barnard and Xue (2004) and Barnard et al. (2002) reported short periods of protection (2 hours) for 7.5% IR3535 in their lab studies.

4) Lemon Eucalyptus (PMD)
Repellents based on this plant chemical provide good protection.Plant-derived repellents can provide good protection against mosquito bites. Those based on lemon eucalyptus provide the longest protection (6-8 hr).
Oil of lemon eucalyptus-based repellents include Repel Lemon Eucalyptus Insect Repellent (20%), and SC Johnson OFF Botanicals (10%). The active ingredient in these repellents is para-menthane3,8-diol (PMD), a chemical that can be extracted from the plant or synthesized. Field studies have shown that 20% PMD provided protection that was equal to 20% DEET but the 10% formulation was not as effective (Carroll and Loye 2006). In laboratory and field studies, the 20% formulations provided protection for 6-8 hr (Barnard and Xue 2004, Carroll and Loye 2006).
5) Soybean oil, Geraniol, or Citronella
Citronella-based products were not effective in laboratory trials. Geraniol-or soybean-oil based products have not been thoroughly tested.
A product containing geraniol (25%, MosquitoSafe) provided 2-4 hr protection in a laboratory study (Barnard and Xue 2004). Oil of citronella contains geraniol as well as citronellal. Field trials have not been published.
Several different citronella-based repellents (5-10%), provided protection for 20 minutes or less in 1 laboratory study (Fradin and Day 2002) and 0.5-5 hr in a second trial (Barnard and Xue 2004). A wristband with 25% citronella failed to prevent bites (Fradin and Day 2002).
2. wear long sleeves, pants, and socks. treat clothes with permethrin.
3. stay in screened or air-conditioned accommodations to keep mosquitoes out.
4. get rid of mosquito sources by emptying standing water from flowerpots, buckets, car tires and barrels.
YELLOW FEVER VACCINE
live attenuated viral vaccine
YF-VAX, the only yellow fever vaccine approved for use in the US, is manufactured by sanofi pasteur.
Recommendations
>=9 months of age who are traveling to or living in areas with risk of yellow fever transmission in South America and Africa.
Some countries require proof of yellow fever vaccination for entry.
Dose and admin
a single injection of 0.5mL should be administrated subcutaneously at 10-year intervals.
Common adverse events
Reported events typically include low-grade fever, headache, and myalgias that begin within days after vaccination and last 5-10 days.
Severe Adverse Events
Hypersensitivity
rash, urticaria or asthma or a combination of these, are uncommon.
1.8 cases/100,000 doses administered.
Yellow fever vaccine-Associated Neurologic Disease(YEL-AND)
meningoencephalitis, Guuillain-Barre syndrome(GBS), acute dissenminated encephalomyelitis(ADEM), bulbar palsy, and Bell's palsy.
YEL-AND was seen primarily among infants as encephalitis.
The onset of illness for ducumented cases ranges 3-28 days after vacccination.
YEL-ANd is rarely fatal.
the incidence of YEL-AND in US is 0.8 per 100,000 doses administered.
Yellow fever Vaccine-Associated Viscerotropic(内脏 ) Disease(YEL-AVD)
it is similar to wild-type disease, with vaccine virus proliferating in multiple organs and oftern leading to MOF and death.
since the initial cases of YEL-AVD were published in 2001, more than 40 confirmed and suspected cases have been reported all over world.
the case-fatality ratio for reported YEL-AVD cases is 53%.
The incidence of YEL-AVD in US is 0.4 cases per 100,000 doses of vaccine administered.
Contraindications
Infants <9 increased risk of postvaccine encephalitis.
the decision to immunize infants who are 6-8 mon of age must balance the infant's risk for exposure with the risk for vaccine-associated encephalitis.
YF vaccine should never be administered to infants <6>
Hypersensitivity.
any of the vaccine components, including gelatin.
The vaccince is produced in chicken embryos. vaccine should not be administered to anyone with a history of acute hypersensitivity to egg or chicken proteins.
Or, desensitiziing and vaccinating procedures are described in the vaccine package insert and should be performed under close medical supervision.
Immunosuppression
symptomatic HIV infection or AIDS, Malignancy, or diseases of the thymus (e.g., thymectomy) or those receiving immunosuppressant therapy(e.g., corticosteroids, alkylating agents, antimetabolites) or radiation therapy.
Immunosuppressed persons should not be immunized and travel to yellow fever-endemic areas should be postponed or avoided. If it is unavoidalbe, persons can not be immunized and if warranted, issued a medical waiver to fulfill internationl health regulations.
Precautions
adults 60 years of age or older
be at increased risk for systemic adverse events compared with younger persons.
Asymptomatic HIV
Persons who are HIV-infected but who do not have AIDS or other sympotomatic manifestations of HIV infection, who have adequate immune system function(e.g., CD4+ cell counts>200/mm3) , and who cannot avoid potential exposure to YFV should be offered the choice of vaccination.
If international travel requirements are the only reason to vaccinate an asymptomatic HIV-infected person, rather than an increased risk for getting YFV, the person should be excused from immunization and isssued amedical waiver to fulfill health regulations.
Pregnancy
women who were vaccinated with YF vaccine early in their pregnancies found no major malformation s in their infants. there was a Slight increased risk noted for minor, mostly skin, malformations.
a higher rate of spontaneous abortions in pregnant women receiving the vaccine was reported but no substantiated.
for pregnant women, if the risk to geting YFV is lower , should be excused from immunization. Or, should be vaccinated, and their infants should be monitored after birth for evidence of congenital infection and other possible adverse effects resulting from YF vaccination.
It is recommended that a woman wait 4 weeks after receiving the live virus yellow fever vaccine before conceiving.(no specific data)
Breastfeeding
Whether the yellow fever vaccine is excreted in breast milk is not known.
Vaccination of nursing mothers should be avoided. However, when travel of nursing mothers to high-risk yellow fever-endemic areas cannot be avoided or postponed, these women should be vaccinated.
Simultaneous Administration of Other Vaccines and Drugs
The immune response to yellow fever vaccine is not inhibited by administration of mealses vaccince(also a live, attenuated vaccine) given concurrently or at various intervals of a few days to 1 mon prior.
recommedation is that injectalbe or nasally administered live vaccines should be given at least 4 weeks apart.
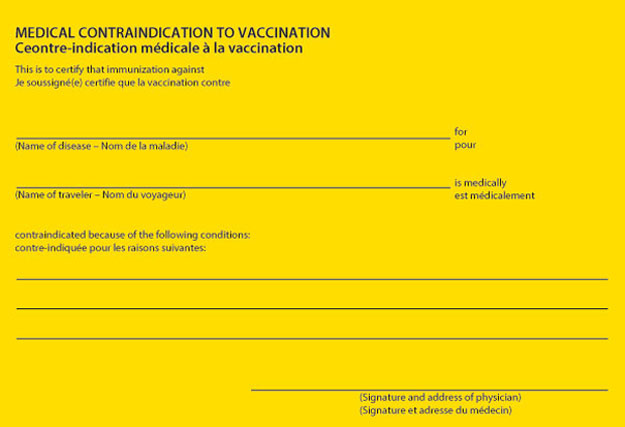
Interatinal Certificate of Vaccination or Prophylaxis(ICVP)
Example International Certificate of Vaccination or Prophylaxis (ICVP)
Example International Certificate of Vaccination or Prophylaxis (ICVP) medical contraindication to vaccination

1 comment:
Thanks for sharing us. home hiv test
Post a Comment